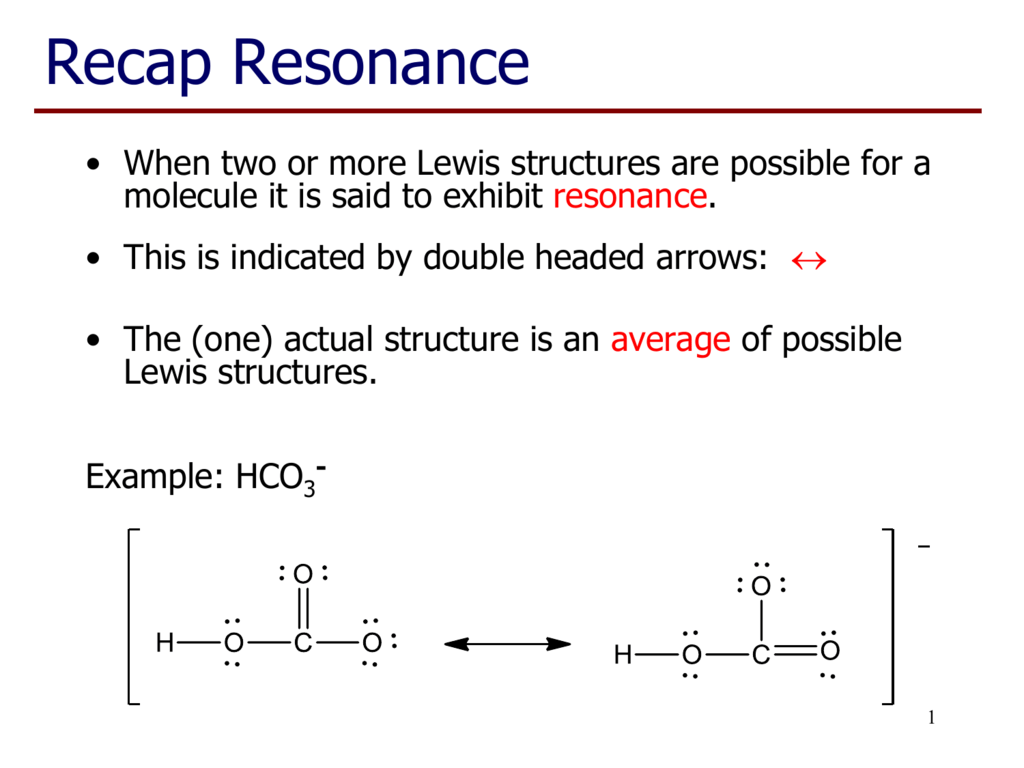
Lewis dot structure for hco3 examquiz
Chemistry 101A Topic F: Molecular Structure 9: Basic Concepts of Covalent Bonding 9.3: Drawing Lewis Structures
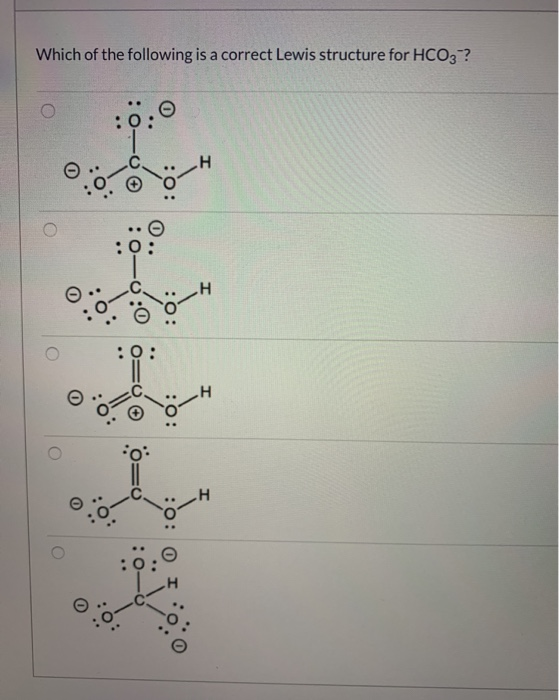
Solved Which of the following is a correct Lewis structure
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

Lewis dot structure for hco3 examquiz
You should put the H CO 3- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge. There are a total of 24 valence electrons in H CO 3-. HCO3- Lewis Structure: How to Draw the Lewis Structure for HCO3- Watch on See the Big List of Lewis Structures
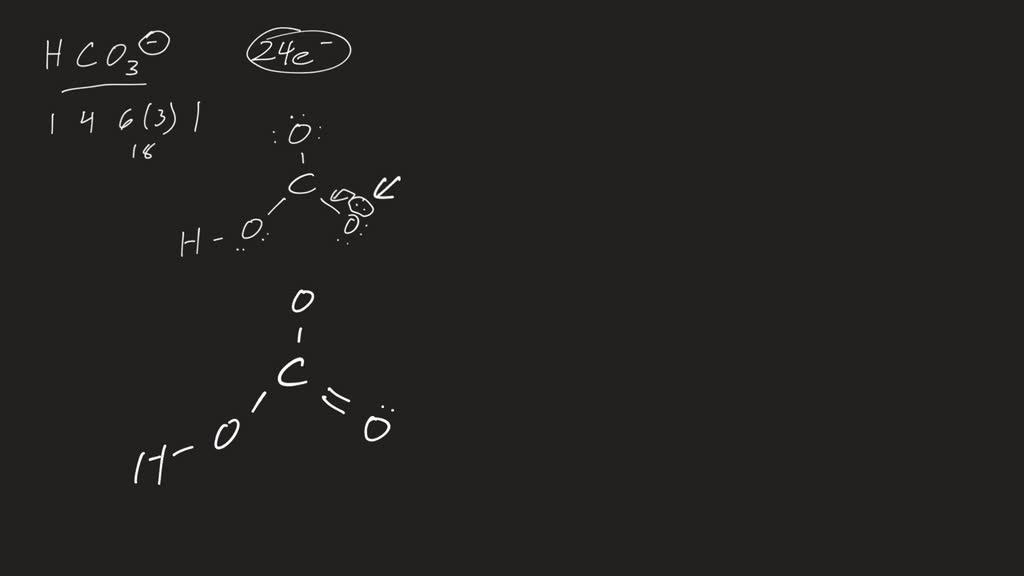
SOLVED(a) The structure of the bicarbonate (hydrogen carbonate) ion
In the lewis structure of carbonic acid (H 2 CO 3 ), carbon atom is the center atom and there are two -OH groups. Also, there is one double bond between carbon and oxygen atoms. As some molecules. there are no lone pairs on carbon atom. From H 2 CO 3 lewis structure, we can say H 2 CO 3 is a dibasic acid. In this tutorial, we will cover how to.
[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion
HCO3- lewis structure has a Carbon atom (C) at the center which is surrounded by two Oxygen atoms (O) and one O-H group. There is 1 double bond between the Carbon atom (C) & Oxygen atom (O) and the rest other atoms have a single bond. There is a -1 formal charge on the single bonded Oxygen atom (O).
Hco3lewis Structure
Drawing the Lewis Structure for H 2 CO 3. When we have an H (or H 2) in front of a polyatomic molecule (like CO 3, SO 4, NO 2, etc.) we know that it's an acid. This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules. For the H 2 CO 3 Lewis structure (Carbonic Acid) make sure you put the Hydrogen atoms on the.

HCO3 Lewis structure, Molecular geometry, Hybridization, Polar or
HCO3- Lewis structure November 7, 2023 by Deep The information on this page is fact-checked. HCO 3- Lewis structure HCO 3- (bicarbonate) has one hydrogen atom, one carbon atom, and three oxygen atoms. In the HCO 3- Lewis structure, there is one double bond and two single bonds around the carbon atom, with three oxygen atoms attached to it.
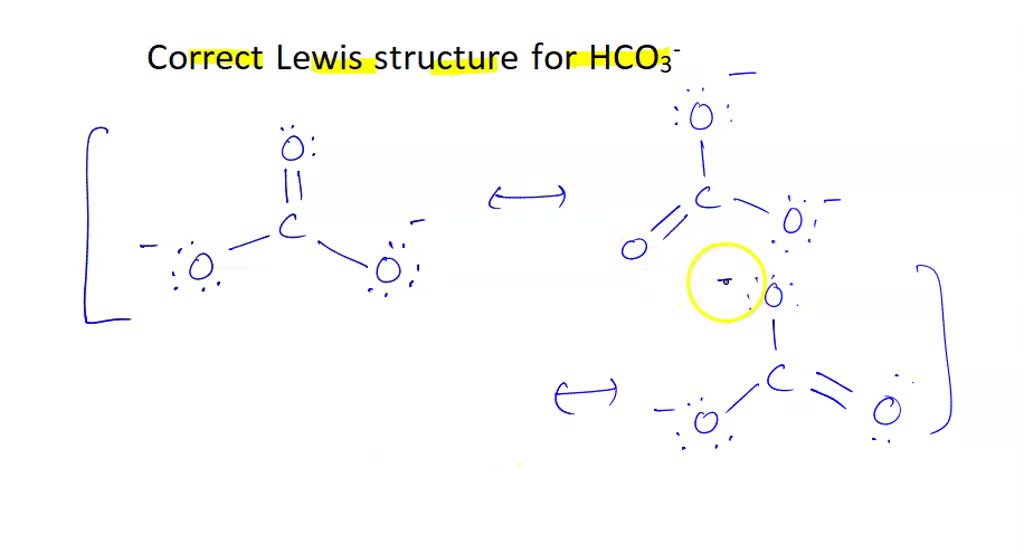
SOLVED Which of the following is the correct Lewis structure for HCO3
Frequency calculation at the HF/6-31G*//HF/6-31G* level showed that the structure 8 is not a minimum, as it contains two imaginary frequencies. $\ce{H3CO3+}$ shares structural similarities with its triaza-analog, the guanidinium ion, as both are possessing resonance stabilization via their onium forms [3, p. 60]. References

Lewis Dot Structure For Sodium
How to draw lewis structure of HCO3-? The bicarbonate (HCO3-) ion comprises a carbon (C) atom at the center. It is double-covalently bonded to an oxygen (O) atom at one side and to another O-atom and an OH functional group vis single covalent bonds at the other two sides. There is no lone pair of electrons on the central C-atom.

HCO3 Molecular Geometry / Shape and Bond Angles YouTube
A step-by-step explanation of how to draw the HCO3- Lewis Dot Structure (Hydrogen Carbonate or Bicarbonate Ion).For the HCO3- structure use the periodic tabl.

Hco3lewis Structure
There are two resonance structures HCO3 - (Bicarbonate ion). We start with a valid Lewis structure and then follow these general rules. For the HCO3 - reson.
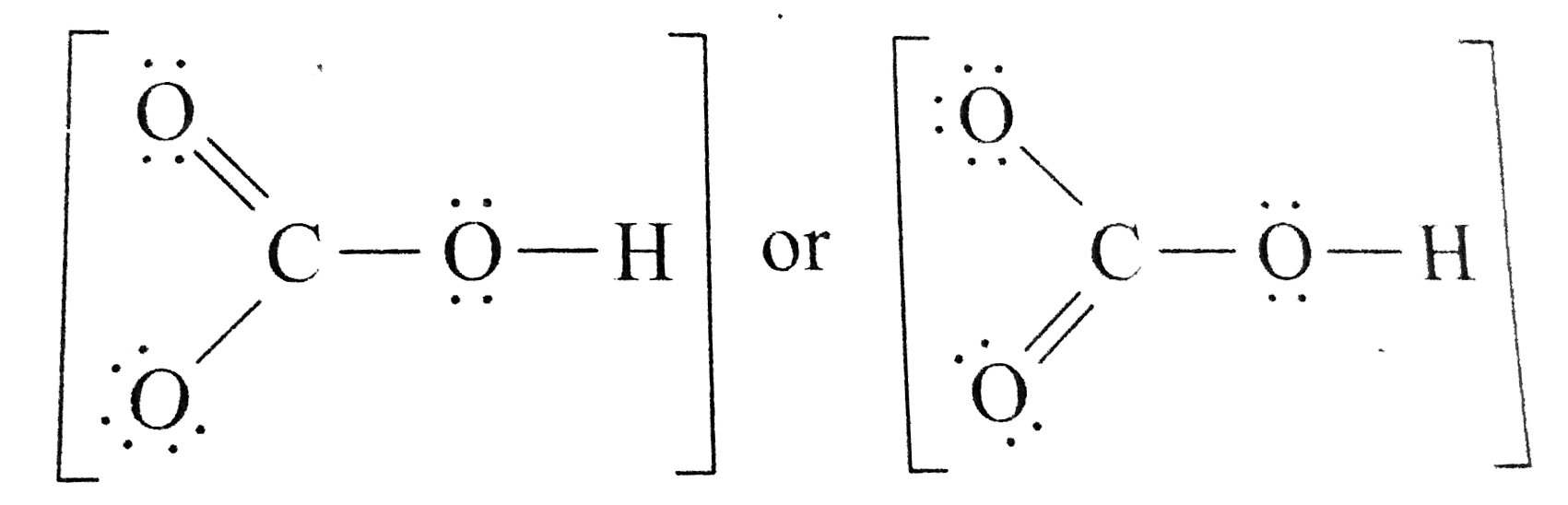
Draw a Lewis structure for the bicarbonate ion, HCO3^().
HCO3- Molecular Geometry / Shape and Bond Angles Wayne Breslyn 725K subscribers Join Subscribe Subscribed 29K views 10 years ago A quick explanation of the molecular geometry of HCO3- including.
[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion
By Sarnali Mukherjee HCO3- Lewis structure is reliable in denoting considerable chemical and physical properties of Bicarbonate. As Lewis structure brings forth a fundamental sketch of HCO3-, it is effective in highlighting the electronic fact about the compound.

How to Calculate the Formal Charges for HCO3 (Bicarbonate ion ) YouTube
Subscribe 661 views 1 year ago Lewis Structure Hello Guys! In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the.
[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion
Check me out: http://www.chemistnate.com
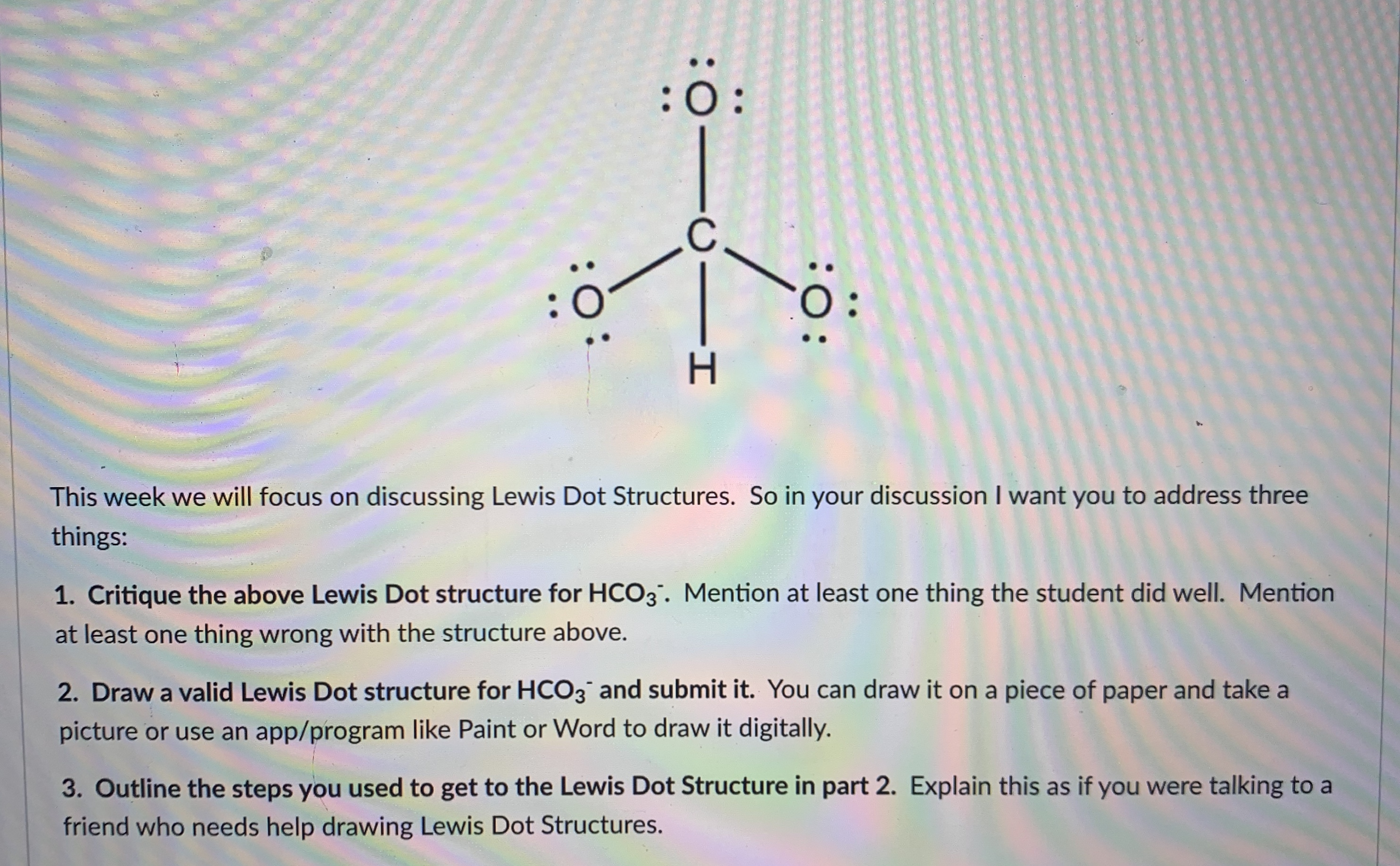
Lewis dot structure for hco3 examquiz
How to Draw the Lewis Structure of Bicarbonate (HCO3-) | Channels for Pearson+ with Jules Exam Prep Explore Next video General Chemistry 11. Bonding & Molecular Structure Lewis Dot Structures: Acids 4m How to Draw the Lewis Structure of Bicarbonate (HCO3-) chemistNATE 888 Was this helpful? 0 Previous video Next video Comments (0) Related Videos